 Gadolinium Neutron Capture Therapy (GdNCT) is a non-invasive experimental therapy for malignant gliomas (brain tumors) never tested on human or animal cases. It is based on a binary approach. In the first step, the patient is intravenously injected with a tumor-seeking compound containing the 157Gd isotope, which has a capture cross section for thermal neutrons many times greater than other elements present in tissue. In the second step, the patient’s skull is exposed to thermal neutrons, which induce a localized, biologically destructive nuclear reaction where Gd is localized.
Gadolinium Neutron Capture Therapy (GdNCT) is a non-invasive experimental therapy for malignant gliomas (brain tumors) never tested on human or animal cases. It is based on a binary approach. In the first step, the patient is intravenously injected with a tumor-seeking compound containing the 157Gd isotope, which has a capture cross section for thermal neutrons many times greater than other elements present in tissue. In the second step, the patient’s skull is exposed to thermal neutrons, which induce a localized, biologically destructive nuclear reaction where Gd is localized.
Gadolinium-157 (157Gd) appears to be a good potential neutron capture agent for several reasons:
- Gd , which is found with a natural abundance of 15.7%, is the most effective isotope in terms of neutron capture, having the largest thermal neutron cross section of all the stable isotopes at 254,000 barn. By comparison, 10B has 3840 barn, 16O has 0.00019 barn, 12C has 0.0035 barn, 1H has 0.333 barn, and 14N has 1.83 barn.
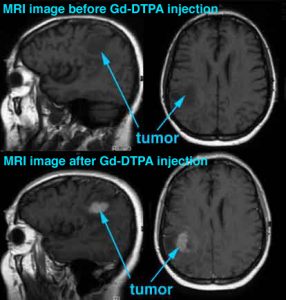
- Some gadolinium compounds are known to accumulate in brain tumors and not in the surrounding healthy tissue. They are in fact used as tumor contrast-enhancing agents for magnetic resonance imaging (MRI), a result of the large magnetic moment of the Gd3+ ion (see figure).
- While the Gd3+ ion is itself toxic, its usefulness in MRI stimulated the search for compounds such as the Gd-DTPA complex, which is stable in the blood stream and non toxic. The pharmacokinetics, biodistribution and tolerance of Gd-DTPA and other Gd compounds used for MRI are well documented.
The gadolinium neutron capture reaction:
157Gd + n = 158Gd* =>158Gd + gamma + x-rays + IC e- + ACK e-
where Gd* is an excited Gd isotope, IC e- are Internal Conversion electrons, and ACK e- are Auger and Coster-Kronig electrons. All the byproducts of this reaction have low linear energy transfer (LET) and induce modest radio-biological damage, with the exception of the high-LET ACK electrons that induce double strand DNA cleavage. These are also the most abundant byproducts (5 ACK electrons per neutron capture). The radiation length of ACK electron is on the order of a few nanometers, therefore their damage is localized. Therefore cancer cell killing by ACK electrons is most effective if they originate from a site within the cell nucleus, i.e. if Gd accumulates intranuclearly.